2024 Influenza and COVID-19 Vaccines Update
Column Author: Christine Symes, MSN, CPNP-PC
Column Editor: Angela L. Myers, MD, MPH | Division Director, Infectious Diseases; Medical Director, Center for Wellbeing
The vaccine recommendations for COVID-19 vaccines for fall 2024 for children 6 months to 4 years of age have been updated. A child in this age group is current on the COVID-19 vaccine when they have received all of the following doses, including at least one dose of the 2024-2025 COVID-19 vaccine. Here are the updated recommendations from the Centers for Disease Control:
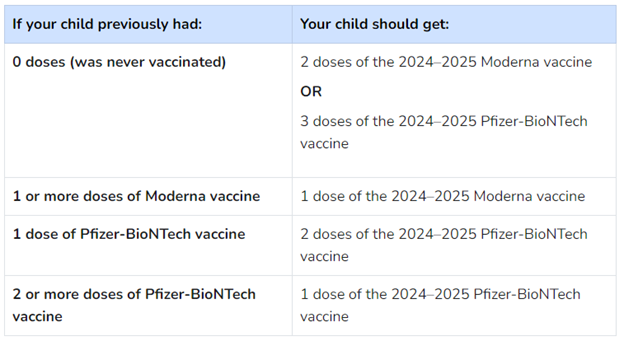
If the same manufacturer is not available or the manufacturer from previous vaccines is unknown, the following recommendations apply:
Administration of COVID-19 vaccine doses from different manufacturers
COVID-19 vaccine doses from the same manufacturer should be administered whenever recommended. In the following circumstances, an age-appropriate COVID-19 vaccine from a different manufacturer may be administered:
- Same vaccine not available at the time of the clinic visit
- Previous dose unknown
- Person would otherwise not receive a recommended vaccine dose
- Person starts but unable to complete a vaccination series with the same COVID-19 vaccine due to a contraindication
A Vaccine Adverse Event Reporting System (VAERS) report is not indicated in these circumstances.
mRNA COVID-19 vaccines
If mRNA vaccine doses are administered from different manufacturers because of a circumstance described above, a three-dose schedule should be followed:
Children ages 6 months to 4 years
- The second dose is administered four to eight weeks after the first dose.
- The third dose of either 2024-2025 Moderna vaccine or 2024-2025 Pfizer-BioNTech vaccine is administered at least eight weeks after the second dose.
Resources:
- Staying up to date with COVID-19 vaccines. Centers for Disease Control and Prevention. September 11, 2024. Accessed September 23, 2024. https://www.cdc.gov/covid/vaccines/stay-up-to-date.html
- Interim clinical considerations for use of COVID-19 vaccines in the United States. Centers for Disease Control and Prevention. Last reviewed September 9, 2024. Accessed September 23, 2024. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html
